Uncategorized
FDA’s Stance on Psychedelic Mushrooms: What You Need to Know
The FDA’s stance on psychedelic mushrooms is evolving rapidly. At Newphoria, we’re closely monitoring these changes and their potential impact on mental health treatments.
Recent developments in FDA-approved research have sparked renewed interest in psilocybin’s therapeutic applications. This blog post will explore the current legal status, ongoing clinical trials, and challenges surrounding FDA approval for psychedelic mushrooms.
What’s the Legal Status of Psychedelic Mushrooms?
FDA Classification and Federal Law
The FDA classifies psilocybin, the active compound in psychedelic mushrooms, as a Schedule I controlled substance. This classification indicates that the FDA considers psilocybin to have a high potential for abuse and no accepted medical use. Federal law prohibits the possession and use of psilocybin mushrooms, with severe penalties (including fines and imprisonment) for violations.
State Laws and Decriminalization Efforts
While federal law remains strict, state laws are changing rapidly. Oregon made history in 2020 by legalizing psilocybin for therapeutic use. The measure is now codified in statute as ORS 475A, which created a two-year development period from January 1, 2021 to December 31, 2022. Colorado followed in 2022, decriminalizing possession and personal use of certain psychedelics, including psilocybin mushrooms.

Several cities have taken steps to decriminalize psilocybin mushrooms:
-
Denver, Colorado (2019)
-
Oakland, California (2019)
-
Santa Cruz, California (2020)
These initiatives typically make the enforcement of laws against personal use and possession of psilocybin mushrooms the lowest priority for law enforcement.
Impact on Research and Therapy
Despite federal prohibition, the FDA has granted “breakthrough therapy” designation to psilocybin for treatment-resistant depression (TRD) and major depressive disorder (MDD) indications. This designation is meant to expedite the development and review of drugs that may offer substantial improvements over existing therapies. It signals that the FDA recognizes the potential therapeutic value of psilocybin, even as it remains a Schedule I substance.
Navigating the Changing Landscape
The legal status of psychedelic mushrooms continues to evolve. As more research emerges and public opinion shifts, we may see further changes in both state and federal laws. It’s crucial to stay informed about local laws and regulations. While the therapeutic potential of psilocybin shows promise, self-medication remains risky and illegal in most jurisdictions.
As we move forward, it’s important to understand how these legal changes affect FDA-approved research on psychedelic mushrooms. Let’s explore the current state of clinical trials and the potential therapeutic applications being studied.
FDA Research on Psychedelic Mushrooms
Groundbreaking Clinical Trials
The FDA’s stance on psychedelic mushrooms continues to evolve as research explores their potential therapeutic applications. Several FDA-approved clinical trials are currently in progress, investigating the use of psilocybin for various mental health conditions.
The Usona Institute conducts a phase 2 clinical trial for major depressive disorder. This study evaluates the safety and efficacy of a 25-mg dose of psilocybin combined with psychological support.

COMPASS Pathways leads a phase 3 clinical trial for treatment-resistant depression. This large-scale study involves over 900 participants across North America and Europe, making it one of the most comprehensive psilocybin trials to date.
Promising Therapeutic Applications
Research indicates that psilocybin may benefit a range of mental health conditions. A study published in the New England Journal of Medicine in 2021 found that psilocybin therapy outperformed escitalopram (a common antidepressant) in treating major depressive disorder.
Johns Hopkins University researchers reported on a randomized clinical trial examining the efficacy of psilocybin as an adjunct to psychotherapy and other treatments for major depressive disorder.
FDA’s Fast-Track Designation
The FDA granted breakthrough therapy designation to psilocybin for both treatment-resistant depression and major depressive disorder. This designation acknowledges psilocybin’s potential to offer substantial improvements over existing therapies and expedites the development and review process.
COMPASS Pathways received this designation for their psilocybin therapy in treatment-resistant depression in 2018. Similarly, Usona Institute was granted breakthrough therapy designation for their psilocybin program in major depressive disorder in 2019.
This fast-track status accelerated research timelines and increased investment in psilocybin studies. However, it’s important to note that while this designation shows promise, it doesn’t guarantee FDA approval. Large-scale clinical trials must still provide rigorous safety and efficacy data before psilocybin can be considered for medical use.
Safety Considerations and Future Outlook
As research progresses, safety remains a top priority. Researchers monitor participants closely for adverse effects and long-term impacts. The FDA requires comprehensive safety data before considering any potential approval for medical use.
The future of psilocybin research looks promising, with more studies planned to explore its potential in treating conditions such as PTSD, anxiety disorders, and eating disorders. As the body of evidence grows, it may reshape our approach to mental health treatment.
While these developments are exciting, it’s essential to remember that psilocybin remains a controlled substance. Self-medication is illegal and potentially dangerous. Always consult healthcare professionals and stay informed about local laws and regulations.
As research continues to unfold, the next challenge lies in addressing the hurdles and controversies surrounding FDA approval for psychedelic substances. Let’s examine these obstacles and their potential impact on the future of psilocybin therapy.
Navigating FDA Approval Hurdles for Psychedelic Mushrooms
Safety Concerns: A Primary Focus
The FDA prioritizes safety in its approval process for psychedelic mushrooms. Extensive data on long-term effects, abuse potential, and drug interactions must be provided. A 2022 Journal of Psychopharmacology study revealed that psilocybin can cause temporary increases in blood pressure and heart rate. These effects, while generally mild, require careful monitoring in clinical settings.
Psychological risks also demand attention. Some participants in psilocybin trials report experiencing anxiety or temporary psychotic-like symptoms. The FDA mandates strict protocols for participant screening and psychological support during and after treatment sessions.
Regulatory Challenges: Navigating the Maze
The Schedule I classification of psilocybin presents a significant obstacle. This designation limits research opportunities and complicates the approval process. Researchers must navigate complex DEA regulations to obtain and handle psilocybin for studies.
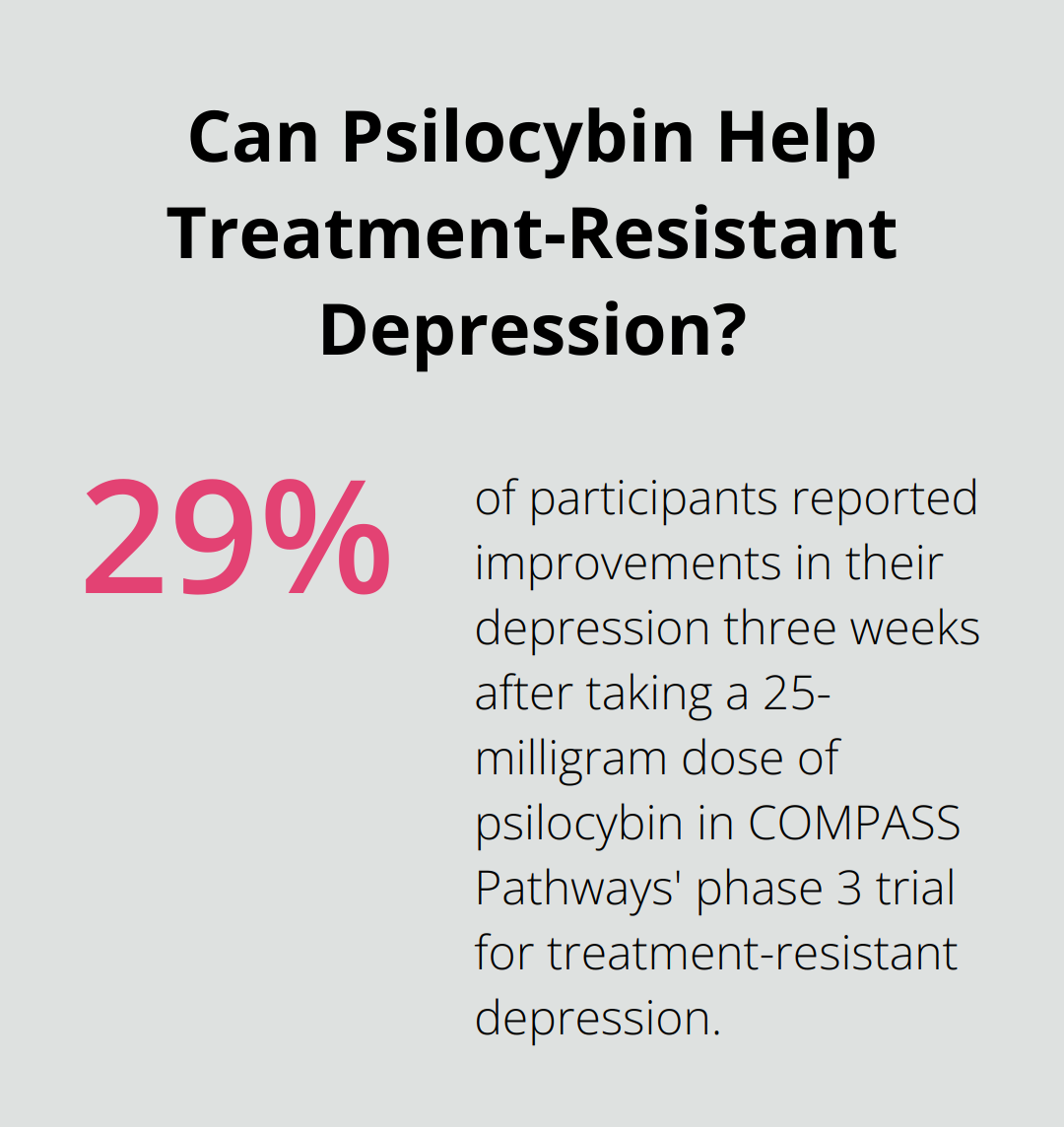
The FDA requires large-scale, multi-center trials, which pose another challenge. These studies are expensive and time-consuming. COMPASS Pathways’ phase 3 trial for treatment-resistant depression exemplifies the scale required for FDA approval. Three weeks after taking a 25-milligram dose of the drug, 29% of participants reported improvements in their depression, the researchers found.
Public Perception: Overcoming Stigma
Public perception of psychedelics remains a hurdle. Despite growing acceptance, many still associate psilocybin with recreational drug use. This stigma can impact funding, participant recruitment, and policy decisions.
Education plays a key role in overcoming this barrier. Organizations work to disseminate accurate information about psychedelics’ therapeutic potential. Their efforts have contributed to a gradual shift in public opinion.
The media significantly shapes perceptions. Responsible reporting on psilocybin research helps combat misinformation. A 2021 analysis in the International Journal of Drug Policy found that media coverage of psychedelics has become more positive and scientifically accurate in recent years.
Balancing Act: Potential Benefits vs. Regulatory Hurdles
The FDA’s evolving stance reflects a growing recognition of psychedelics’ therapeutic potential. This signals a possible shift in the regulatory landscape. However, the approval process remains rigorous (as it should).
Researchers and advocates must balance the potential benefits of psilocybin therapy with the need for thorough safety evaluations. This balance ensures that any approved treatments meet the highest standards of efficacy and safety.
Final Thoughts
The FDA’s stance on psychedelic mushrooms continues to evolve, reflecting a growing recognition of their potential therapeutic benefits. Despite psilocybin’s current Schedule I status, the FDA has shown openness to research through breakthrough therapy designations and approved clinical trials. This shift signals a potential future where psychedelic-assisted therapies could become part of mainstream mental health treatment.

The landscape of psychedelic research and policy changes rapidly. Healthcare professionals, patients, and the general public must stay informed about these evolving policies. As new studies emerge and regulations shift, it’s important to rely on credible sources for up-to-date information on FDA psychedelic mushrooms research and policy changes.
While the therapeutic potential of psychedelics shows promise, their use must occur responsibly and within legal frameworks. For those interested in exploring psychedelics safely and legally, Newphoria offers high-quality products, including mushrooms and other psychedelics, with a focus on safety and accessibility. As research progresses, we at Newphoria remain committed to providing accurate information and quality products to support the evolving landscape of psychedelic therapy.

